New method for bicyclo[1.1.1]pentane ketones synthesis
GA, UNITED STATES, March 26, 2025 /EINPresswire.com/ -- We present a one-step, visible light-induced synthesis of bicyclo[1.1.1]pentane-ketone, a bioisostere for benzoyl groups that enhances drug properties. This method operates under mild conditions, tolerates oxidation-sensitive substituents, and achieves moderate to high yields. Mechanistic studies indicate the necessity of stoichiometric tBuOOH despite the reaction's redox-neutral nature. This approach offers a streamlined strategy for incorporating bicyclo[1.1.1]pentane-ketone into pharmaceuticals, potentially improving their efficacy and compatibility.
Since the 1990s, medicinal chemistry research has demonstrated that replacing benzene rings with bicyclo[1.1.1]pentane derivatives enhances drug properties such as solubility and metabolic stability while also circumventing patent restrictions. Notably, in 2009, Frank Lovering introduced the "escape from planarity" concept, which has gained momentum as research explores substituting planar aromatic hydrocarbons with three-dimensional cyclo[n.1.1]alkanes.
Benzoyl groups are prevalent in pharmaceuticals, making the replacement of benzene rings with bicyclo[1.1.1]pentane an intriguing area of study. However, existing synthetic methods often required high temperatures, metal catalysts or hazardous reagents.
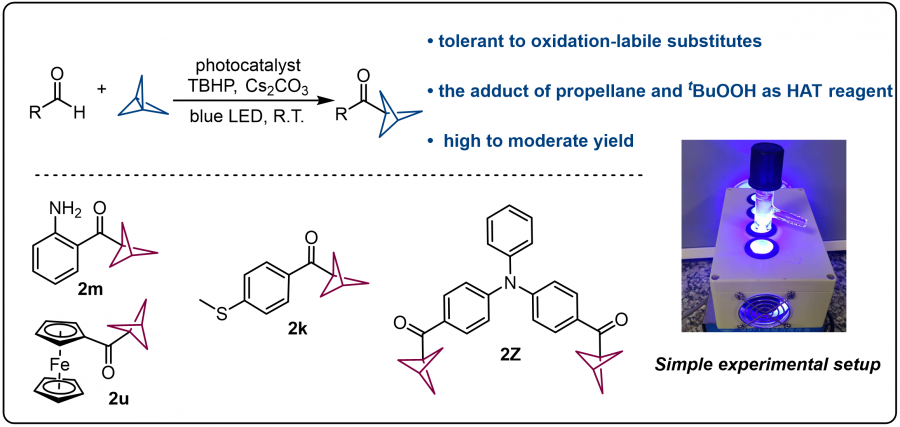
In a new study, a team of researchers in China employed tert-butyl hydrogen peroxide (TBHP) as a hydrogen transfer agent under blue light to activate aldehyde hydrogen bonds, generating acyl radicals that react with spiral alkanes to form bicyclo[1.1.1]pentane-ketones (BCP-ketones).
"This mild, metal-free method proceeds at room temperature with moderate to high yields and tolerates oxidation-sensitive groups such as amino, methylthio, and ferrocene (2m, 2k, 2u)," shares Fener Chen, senior and corresponding author of the study. "In particular, we synthesized a molecule being incorporated of two BCP rings for the first time (2z)."
Mechanistic studies confirmed that reducing TBHP to catalytic amounts halted the reaction, indicating its stoichiometric consumption. High-resolution mass spectrometry and radical trapping experiments further supported the involvement of acyl radicals, validating a radical-based mechanism.
"In summary, we developed a one-step visible light-induced approach for the synthesis of bicyclo[1.1.1]pentane-ketone, characterized by mild reaction temperature and excellent tolerance to oxidation-labile substituents, delivering all products in moderate to high yields," adds Chen.
References
DOI
10.1016/j.gresc.2025.03.003
Original Source URL
https://doi.org/10.1016/j.gresc.2025.03.003
Funding information
This work was financially supported by the National Natural Science Foundation of China (No. 22208056), and Major Program of Qingyuan Innovation Laboratory (No. 00122001)
Lucy Wang
BioDesign Research
email us here
Distribution channels: Chemical Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release