Coloplast Altis Sling: Supplemental General Causation Expert Reports Served
Concerns raised over Altis sling as studies show higher rates of pain and complications compared to other mid-urethral slings
SANTA BARBARA, CA, UNITED STATES, May 12, 2025 /EINPresswire.com/ -- “We believe that no reasonable physician would choose the Altis sling when informed of what Coloplast knows about its risks," states Greg Vigna, MD, JD, national mid-urethral sling attorney.
Greg Vigna, MD, JD, malpractice and product liability attorney, states, “In the coming days, several themes will emerge regarding the Altis device, which we allege is the most harmful device on the market in terms of pain. No reasonable doctor would select this device if they were aware of what Coloplast knows, and no reasonable doctor would delay complete mesh removal in patients experiencing disabling leg pain, thigh pain, hip pain, and dyspareunia following implantation."
Dr. Vigna continues, “We have themes that are supported by Coloplast sponsored studies, internal documents, and Level 1 Evidence from the SIMS trial. My firm’s position is that no woman would elect to have this device if fully informed of its substantial risk of pain compared to other mid-urethral slings."
YIKES 1: The Altis device is too stiff.
“The structurally stiffer Altis sling had decreased tissue integration and increased propensity to buckle after implantation. Increase collagen types I and III after the implantation of this device suggests that these changes may be associated with a fibrotic response. In contrast, the Solyx sling largely maintained a flat configuration and had improved tissue integration. The deformation of the Altis sling is not an intended effect and is likely caused by its lower bending stiffness,” states Dr. Moalli.
Read Dr. Pamela Moalli’s study comparing the Altis to the Solyx in sheep: https://www.sciencedirect.com/science/article/abs/pii/S0002937820307225
YIKES 2: The Altis causes dyspareunia in 20% of women at 15 months. (See Table 17: Summary of AEs, Dyspareunia).
Read the SIMS trial (Health Technology Assessment, No. 26): https://www.ncbi.nlm.nih.gov/books/NBK587586
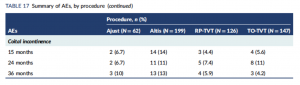
YIKES 3: The Altis causes coital incontinence 2-3x that of full-length mid-urethral slings. That is involuntary incontinence during sex. (See Table 17, Summary of AEs, Coital incontinence).
Read the SIMS trial (Health Technology Assessment, No. 47): https://www.ncbi.nlm.nih.gov/books/NBK587586
YIKES 4: The Coloplast sponsored Altis 522 Study at 3-years shows the occurrence of inner leg and thigh pain in frequencies that are unique to this stiff device. The comparator are full length slings. (See Table 4: Extremity pain).
Read the Altis 522 Study: https://onlinelibrary.wiley.com/doi/pdf/10.1002/nau.25256
Dr. Vigna continues, “This pain is not from positioning. This is acute pain caused by the stiff device. To make matters worse, 23.9% of the women implanted with the Altis did not complete the study.”
YIKES 5: Coloplast has known since the Investigational Device Exemption study that the Coloplast causes pain in at least 10% of women.
“The most common procedure- and device-related adverse events consisted of hip/groin pain (7.1%, 8/113), mesh extrusion (3.5%, 4/113), pelvic/urogenital pain (3.5%, 4/113).”
Read the Poster: https://www.ics.org/Abstracts/Publish/218/000633.pdf
Dr. Vigna concludes, “Reassurance by implanting physicians is not a treatment plan when early pain or dyspareunia occurs following implantation of this stiff device.”
The Vigna Law Group is investigating the Red Flag Warning symptoms of neurological injury from the Coloplast Altis sling, including:
“Other: Non-pelvic pain” including anatomic groin pain (inner leg pain), thigh pain, hip pain.
“Pelvic/Urogenital (groin) pain”: Pain not including the inner leg, thigh, or hip including:
1. Inability to wear tight pants
2. Clitoral pain or numbness
3. Severe pain that makes vaginal penetration impossible
4. Tailbone pain
5. Anorectal pain
6. Painful bladder
7. Pain with sitting
Dr. Vigna is a California and Washington DC lawyer who focuses on catastrophic pain syndromes caused by the Coloplast Altis sling, including pudendal neuralgia and obturator neuralgia. He represents women with the Ben Martin Law Group, a national pharmaceutical injury law firm in Dallas, Texas. The attorneys are product liability and medical malpractice attorneys, and they represent neurological injuries across the country.
Click here for a free book on Vaginal Mesh Pain.
Greg Vigna, MD, JD
Vigna Law Group
+1 8178099023
email us here
Visit us on social media:
LinkedIn
Facebook
X
Distribution channels: Business & Economy, Education, Healthcare & Pharmaceuticals Industry, Law, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release