
Moderate to Severe Atopic Dermatitis Clinical Trial Pipeline Insights Featuring 45+ Companies | DelveInsight
The moderate to severe atopic dermatitis market is experiencing strong growth driven by rising global prevalence and heightened awareness, fueling demand for advanced treatments. Breakthroughs in biologics, small molecules, and personalized medicine are reshaping the therapeutic landscape. A robust pipeline, frequent drug approvals, and supportive reimbursement frameworks further enhance market momentum. Additionally, innovations in pediatric care and improved global access continue to expand opportunities in this evolving space.
/EIN News/ -- New York, USA, May 20, 2025 (GLOBE NEWSWIRE) -- Moderate to Severe Atopic Dermatitis Clinical Trial Pipeline Insights Featuring 45+ Companies | DelveInsight
The moderate to severe atopic dermatitis market is experiencing strong growth driven by rising global prevalence and heightened awareness, fueling demand for advanced treatments. Breakthroughs in biologics, small molecules, and personalized medicine are reshaping the therapeutic landscape. A robust pipeline, frequent drug approvals, and supportive reimbursement frameworks further enhance market momentum. Additionally, innovations in pediatric care and improved global access continue to expand opportunities in this evolving space.
DelveInsight’s 'Moderate to Severe Atopic Dermatitis Pipeline Insight 2025' report provides comprehensive global coverage of pipeline moderate to severe atopic dermatitis therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the moderate to severe atopic dermatitis pipeline domain.
Key Takeaways from the Moderate to Severe Atopic Dermatitis Pipeline Report
- DelveInsight’s moderate-to-severe atopic dermatitis pipeline report depicts a robust space with 45+ active players working to develop 50+ pipeline moderate-to-severe atopic dermatitis drugs.
- Key moderate to severe atopic dermatitis companies such as Jiangsu Vcare Pharmaceutical Technology Co., LTD, Shanghai Chia Tai Tianqing Pharmaceutical Technology Development Co., Ltd., Nektar Therapeutics, E-nitiate Biopharmaceuticals (Hangzhou) Co., Ltd., Corvus Pharmaceuticals, Inc., UCB Biopharma SRL, Tavotek Biotherapeutics, Guangdong Hengrui Pharmaceutical Co., Ltd, SCM Lifescience Co., LTD., Sanofi, Amgen, Qurient Co., Ltd., Sun Pharmaceutical Industries Limited, Pfizer, UNION therapeutics, Bio-Thera Solutions, medac GmbH, Eli Lilly and Company, AbbVie, Lynk Pharmaceuticals Co., Ltd, LEO Pharma, Guangzhou JOYO Pharma Co., Ltd, Beijing InnoCare Pharma Tech Co., Ltd., Oneness Biotech Co., Ltd., Evommune, Inc., Apogee Therapeutics, Kymera Therapeutics, and others are evaluating new moderate to severe atopic dermatitis drugs to improve the treatment landscape.
- Promising pipeline moderate to severe atopic dermatitis therapies such as VC005, TQH2722, Rezpegaldesleukin, QY201, Soquelitinib, UCB9741, TAVO101, SHR-1819, SCM-AGH, SAR444656, Rocatinlimab, Q301, SCD-044, PF-07832837, Orismilast, BAT6026, Methotrexate, LY3454738, Lutikizumab, LNK01001, LEO 138559, JYP0061, ICP-332, FB825, EVO301, APG777, KT-621, and others are in different phases of moderate to severe atopic dermatitis clinical trials.
- In April 2025, Kymera Therapeutics announced that it had recently initiated dosing in its BroADen Phase Ib clinical trial evaluating KT-621, an oral, highly selective, potent degrader of STAT6, in patients with moderate to severe atopic dermatitis (AD). The Company expects to report data from the BroADen trial in the fourth quarter of 2025.
- In April 2025, Corvus Pharmaceuticals announced that new interim data from the randomized, double-blind, placebo-controlled Phase I clinical trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis will be presented in an oral session and poster at the Society for Investigative Dermatology.
- In February 2025, Apogee Therapeutics announced that the first patient had been dosed in the Part B portion of the Phase II APEX clinical trial of APG777 in patients with moderate-to-severe AD, as well as enrollment completion in the Part A portion of the trial.
- In February 2025, the FDA granted fast-track designation to rezpegaldesleukin for the treatment of patients 12 years and older with moderate-to-severe atopic dermatitis whose disease is not controlled with topical prescription therapies or for which those therapies are not advisable.
- In January 2025, Nektar Therapeutics announced that the company had completed target enrollment in its REZOLVE-AD Phase IIb study of rezpegaldesleukin in patients with moderate-to-severe atopic dermatitis.
- In December 2024, Corvus Pharmaceuticals announced interim data from the randomized, double-blind, placebo-controlled Phase I clinical trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis.
- In December 2024, Celldex Therapeutics announced that the Company has initiated a Phase II study of barzolvolimab in moderate to severe atopic dermatitis and that the study is actively enrolling patients.
- In October 2024, LEO Pharma presented the final results of the five-year extension study ECZTEND, showing the long-term safety and efficacy profile of Adbry® (tralokinumab-ldrm) in adults and adolescent patients (aged 12 years and over) with moderate-to-severe atopic dermatitis (AD).
- In January 2024, Aclaris Therapeutics announced top-line results from its Phase IIb study of ATI-1777, an investigational topical “soft” JAK 1/3 inhibitor, in patients with mild to severe atopic dermatitis (AD) (ATI-1777-AD-202; NCT05432596). ATI-1777 was generated from Aclaris’ proprietary KINect® drug discovery platform.
Request a sample and discover the recent advances in moderate to severe atopic dermatitis drugs @ Moderate to Severe Atopic Dermatitis Pipeline Report
The moderate to severe atopic dermatitis pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage moderate to severe atopic dermatitis drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the moderate to severe atopic dermatitis clinical trial landscape.
Moderate to Severe Atopic Dermatitis Overview
Atopic dermatitis is the most prevalent chronic inflammatory skin condition, often starting in infancy and marked by dry skin, eczema-like lesions, and thickened or leathery patches. It frequently occurs alongside other IgE-mediated conditions, such as asthma, allergic rhinitis, and food allergies. Over the last few decades, the incidence of atopic dermatitis has been on the rise, contributing to a high burden of disease. Moderate to severe cases are characterized by frequent flare-ups, intense itching, and skin changes like discoloration and raised, rough patches. In severe cases, persistent nighttime itching can disrupt sleep and daily life, worsening overall health and well-being. Beyond physical symptoms, atopic dermatitis can take a toll on mental health, often leading to anxiety, depression, and social discomfort due to the visible nature of the skin condition. Effective treatment typically involves a mix of skincare practices, medications, and lifestyle adjustments to manage flare-ups and improve patients' quality of life.
The underlying causes of moderate to severe atopic dermatitis are multifactorial, involving both genetic predispositions and environmental influences that disrupt skin barrier function and immune regulation. atopic dermatitis is part of the “atopic triad” along with asthma and allergic rhinitis, and often progresses through the “atopic march,” where symptoms develop sequentially over time. Many individuals with atopic dermatitis carry mutations in the filaggrin gene, which is vital for maintaining skin hydration, and these mutations may also increase susceptibility to other allergic conditions. Environmental triggers such as exposure to allergens like eggs, dairy, peanuts, and cigarette smoke can worsen symptoms, making disease control more complex. These genetic and environmental contributors explain the chronic and sometimes disabling nature of moderate to severe atopic dermatitis.
Moderate to severe atopic dermatitis presents with a variety of persistent and uncomfortable symptoms, including severe itching, red or discolored patches, and dry, flaky skin. Affected areas may crack, swell, ooze, and develop crusts. In more severe cases, symptoms can affect the entire body or flare up in localized areas such as the face, eyelids, neck, scalp, lips, ears, hands, feet, and behind the knees. The skin often becomes extremely sensitive, further intensifying discomfort and negatively affecting daily life.
Managing moderate to severe atopic dermatitis requires a comprehensive and personalized treatment strategy. This typically includes identifying and avoiding triggers, maintaining a consistent skincare routine, and using anti-inflammatory therapies. Daily moisturization is crucial—emollients should be applied twice daily, especially within three minutes after bathing, to lock in moisture. Topical corticosteroids are commonly used to control acute flare-ups, while nonsteroidal options like calcineurin inhibitors are preferred for delicate areas. When topical treatments are inadequate, systemic options such as phototherapy or immunosuppressive drugs like cyclosporine may be used.
Biologic therapies, notably dupilumab, target specific immune pathways by inhibiting IL-4 and IL-13 signaling and have shown significant benefits. Additional supportive treatments include dilute bleach baths to reduce skin bacterial load and the use of probiotics during pregnancy and breastfeeding to potentially lower the incidence of atopic dermatitis in children. Furthermore, crisaborole, a topical phosphodiesterase-4 (PDE-4) inhibitor, provides a non-steroidal option for treating mild to moderate forms of the disease.
Find out more about moderate to severe atopic dermatitis drugs @ Moderate to Severe Atopic Dermatitis Treatment
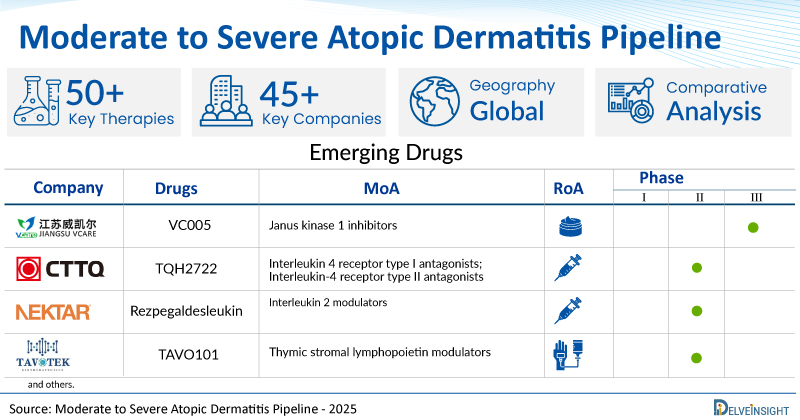
A snapshot of the Pipeline Moderate to Severe Atopic Dermatitis Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| VC005 | Jiangsu Vcare Pharmaceutical Technology Co., LTD | III | Janus kinase 1 inhibitors | Topical |
| TQH2722 | Shanghai Chia Tai Tianqing Pharmaceutical Technology Development Co., Ltd. | II | Interleukin 4 receptor type I antagonists; Interleukin-4 receptor type II antagonists | Subcutaneous |
| Rezpegaldesleukin | Nektar Therapeutics | II | Interleukin 2 modulators | Subcutaneous |
| TAVO101 | Tavotek Biotherapeutics | II | Thymic stromal lymphopoietin modulators | Intravenous |
| QY201 | E-nitiate Biopharmaceuticals (Hangzhou) Co., Ltd. | II | Janus kinase 1 inhibitors; TYK2 kinase inhibitors | Oral |
| UCB9741 | UCB Biopharma | I/II | Immunomodulators | Intravenous |
| Soquelitinib | Corvus Pharmaceuticals, Inc. | I | Emt protein-tyrosine kinase inhibitors | Oral |
Learn more about the emerging moderate to severe atopic dermatitis therapies @ Moderate to Severe Atopic Dermatitis Clinical Trials
Moderate to Severe Atopic Dermatitis Therapeutics Assessment
The moderate to severe atopic dermatitis pipeline report proffers an integral view of the emerging moderate to severe atopic dermatitis therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Moderate to Severe Atopic Dermatitis Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Janus kinase 1 inhibitors, Interleukin 4 receptor type I antagonists, Interleukin-4 receptor type II antagonists, Interleukin 2 modulators, TYK2 kinase inhibitors, Emt protein-tyrosine kinase inhibitors, Immunomodulators, Thymic stromal lymphopoietin modulators, Interleukin 4 receptor alpha subunit antagonists
- Key Moderate to Severe Atopic Dermatitis Companies: Jiangsu Vcare Pharmaceutical Technology Co., LTD, Shanghai Chia Tai Tianqing Pharmaceutical Technology Development Co., Ltd., Nektar Therapeutics, E-nitiate Biopharmaceuticals (Hangzhou) Co., Ltd., Corvus Pharmaceuticals, Inc., UCB Biopharma SRL, Tavotek Biotherapeutics, Guangdong Hengrui Pharmaceutical Co., Ltd, SCM Lifescience Co., LTD., Sanofi, Amgen, Qurient Co., Ltd., Sun Pharmaceutical Industries Limited, Pfizer, UNION therapeutics, Bio-Thera Solutions, medac GmbH, Eli Lilly and Company, AbbVie, Lynk Pharmaceuticals Co., Ltd, LEO Pharma, Guangzhou JOYO Pharma Co., Ltd, Beijing InnoCare Pharma Tech Co., Ltd., Oneness Biotech Co., Ltd., Evommune, Inc., Apogee Therapeutics, Kymera Therapeutics, and others.
- Key Moderate to Severe Atopic Dermatitis Pipeline Therapies: VC005, TQH2722, Rezpegaldesleukin, QY201, Soquelitinib, UCB9741, TAVO101, SHR-1819, SCM-AGH, SAR444656, Rocatinlimab, Q301, SCD-044, PF-07832837, Orismilast, BAT6026, Methotrexate, LY3454738, Lutikizumab, LNK01001, LEO 138559, JYP0061, ICP-332, FB825, EVO301, APG777, KT-621, and others.
Dive deep into rich insights for new moderate to severe atopic dermatitis treatments, visit @ Moderate to Severe Atopic Dermatitis Drugs
Table of Contents
| 1. | Moderate to Severe Atopic Dermatitis Pipeline Report Introduction |
| 2. | Moderate to Severe Atopic Dermatitis Pipeline Report Executive Summary |
| 3. | Moderate to Severe Atopic Dermatitis Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Moderate to Severe Atopic Dermatitis Clinical Trial Therapeutics |
| 6. | Moderate to Severe Atopic Dermatitis Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Moderate to Severe Atopic Dermatitis Pipeline: Late-Stage Products (Phase III) |
| 8. | Moderate to Severe Atopic Dermatitis Pipeline: Mid-Stage Products (Phase II) |
| 9. | Moderate to Severe Atopic Dermatitis Pipeline: Early-Stage Products (Phase I) |
| 10. | Moderate to Severe Atopic Dermatitis Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Moderate to Severe Atopic Dermatitis Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Moderate to Severe Atopic Dermatitis Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the moderate to severe atopic dermatitis pipeline therapeutics, reach out @ Moderate to Severe Atopic Dermatitis Therapeutics
Related Reports
Moderate to Severe Atopic Dermatitis Market
Moderate to Severe Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key moderate to severe atopic dermatitis companies, including Jiangsu Vcare Pharmaceutical Technology Co., LTD, Shanghai Chia Tai Tianqing Pharmaceutical Technology Development Co., Ltd., Nektar Therapeutics, E-nitiate Biopharmaceuticals (Hangzhou) Co., Ltd., Corvus Pharmaceuticals, Inc., UCB Biopharma SRL, Tavotek Biotherapeutics, Guangdong Hengrui Pharmaceutical Co., Ltd, SCM Lifescience Co., LTD., Sanofi, Amgen, Qurient Co., Ltd., Sun Pharmaceutical Industries Limited, Pfizer, UNION therapeutics, among others.
Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atopic dermatitis companies, including Arcutis Biotherapeutics, Amgen, Kyowa Kirin, Dermavant Sciences, Cara Therapeutics, Pfizer, Arena Pharmaceuticals, BioMimetix, Eli Lilly and Company, Aldeyra Therapeutics, Inc., Hangzhou Yirui Pharmaceutical Technology Co., Ltd, LEO Pharma, Corvus Pharmaceuticals, Inc., Brexogen Inc., Sanofi, Shaperon, UCB Pharma, Q32 Bio Inc., Akeso, Apogee Therapeutics, Inc., Allakos Inc., Biosion, Inc., among others.
Atopic Dermatitis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key atopic dermatitis companies, including Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, Dermira, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, and others. Kymab, BiomX, LEO Pharma, GlaxoSmithKline, Arjil Pharmaceuticals, SCM Lifescience, Sun Pharmaceutical Industries Limited, Brickell Biotech Inc, AstraZeneca, Kyowa Kirin, UCB Biopharma, Arcutis Biotherapeutics, Vanda Pharmaceuticals, Kyowa Kirin, Sanofi, KeyMed Biosciences, Asana BioSciences, Bristol-Myers Squibb, RAPT Therapeutics, Allakos, Novartis, BioMimetix, Shanghai Hengrui Pharmaceutical Co, Connect Biopharma, Pfizer, Evommune, Inc., Fresh Tracks Therapeutics, Biosion, Chia Tai Tianqing Pharmaceutical, Reistone Biopharma Company Limited, JW Pharmaceutical, Oneness Biotech, Alphyn Biologics, selectION, UNION Therapeutics, Ichnos Scien, among others.
Severe Atopic Dermatitis Market
Severe Atopic Dermatitis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key severe atopic dermatitis companies, including Organon, Roche, Eli Lilly and Company, Chugai Pharmaceuticals, AbbVie, Pfizer, LEO Pharma, Otsuka Pharmaceutical, Incyte Corporation, Kyowa Kirin, Amgen, Sanofi, UNION Therapeutics, Aclaris Therapeutics, Sun Pharma, Connect Biopharma, among others.
Atopic Dermatitis Epidemiology Forecast
Atopic Dermatitis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted atopic dermatitis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

