
Caught in the Actinium
One area of interest is in using an isotope of actinium (actinium-225) in a cancer treatment method called targeted alpha therapy (TAT), which has shown promise in clinical trials. The TAT method uses biological delivery systems such as peptides or antibodies to move the radioactive element to the cancer site. When the actinium decays, it releases energetic particles that travel a short distance, destroying the nearby cancer cells but sparing healthy tissue further away.
“There’s a movement to design better delivery systems to get the actinium to particular cells and keep it there,” said Rebecca Abergel, a UC Berkeley associate professor of nuclear engineering and of chemistry who leads the Heavy Element Chemistry Group at Berkeley Lab. “If we can engineer proteins to bind the actinium with a really high affinity, and either be fused with an antibody or serve as the targeting protein, that would really enable new ways to develop radiopharmaceuticals.”
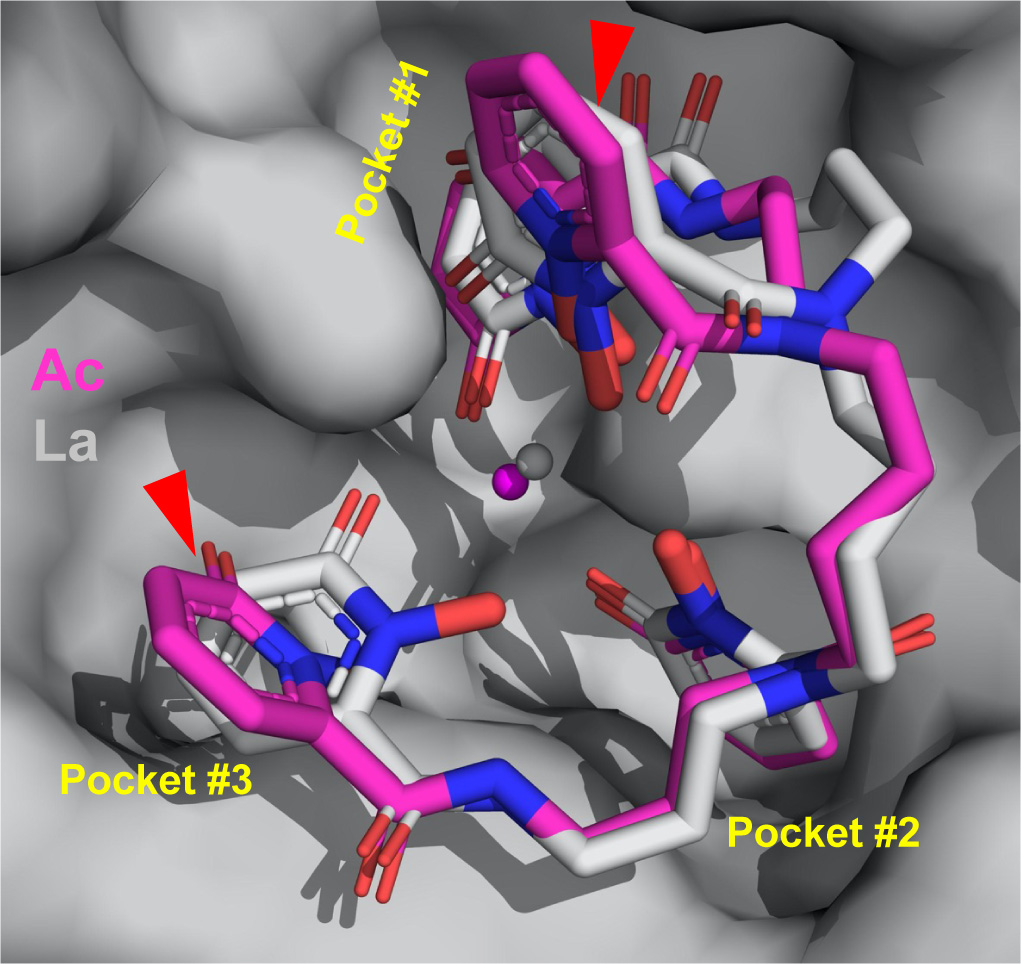
This rendering shows the structure of how actinium (magenta) binds with other molecules. Red triangles point out how the arrangement differs from actinium’s lighter counterpart, lanthanum (grey). The stick structure of the binding molecule (the ligand) is surrounded by pockets in the protein. (Credit: Jen Wacker/Berkeley Lab)
Researchers used a novel approach to grow the crystals using only 5 micrograms of pure actinium – roughly one tenth the weight of a grain of salt, and invisible to the naked eye. They first purified the actinium through a complex filtration process that removed other elements and chemical impurities. They then bound the actinium to a metal-trapping molecule called a ligand and enveloped the bundle inside of a protein isolated and purified by Roland Strong’s team at the Fred Hutchinson Cancer Center, building a “macromolecular scaffold.” The crystals, grown over a week inside of the Heavy Element Research Laboratory, were then cryocooled in liquid nitrogen and illuminated with X-rays at Berkeley Lab’s Advanced Light Source (ALS). The X-rays revealed the compound’s 3D structure and showed how actinium interacted with surrounding atoms. It is the first single-crystal X-ray structure reported for actinium.
“I’ve been working in crystallography for 40 years and seen a lot of things, and the method the team is using is unique and provides details we couldn’t get in the past,” said Marc Allaire, a scientist in Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division and head of the Berkeley Center for Structural Biology team at the ALS. “To the best of my knowledge, Berkeley Lab is the only place in the world where we do this kind of study and measure radioactive protein crystals.”
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.

