Bi-superlattice strategy unlocks high-energy, long-life sodium-ion batteries
GA, UNITED STATES, May 23, 2025 /EINPresswire.com/ -- A new study reports a promising advance in sodium-ion battery technology by achieving highly reversible cationic and anionic redox reactions—key to unlocking longer cycle life and higher energy density. Researchers developed a honeycomb-layered cathode material, Na₀.₇₈Ni₀.₁₂Li₀.₁₈Mn₀.₇O₂ (NNLMO), featuring a dual-topology NiMn₆/LiMn₆ superlattice. This design effectively stabilizes the oxygen redox process and mitigates structural degradation. As a result, the battery delivers a reversible capacity of 224 mAh g⁻¹ and retains over 92% capacity after 50 cycles. The findings offer a new structural strategy for advancing sodium-based energy storage systems.
Sodium-ion batteries (SIBs) are increasingly recognized as a cost-effective and sustainable alternative to lithium-ion batteries, particularly for large-scale energy storage. However, achieving high energy density while maintaining structural and electrochemical stability remains a major challenge. Cathode materials based on layered transition metal oxides with oxygen anionic redox activity offer enhanced capacity but often suffer from irreversible oxygen loss, voltage hysteresis, and phase transitions. These issues stem from cation migration and unstable oxygen coordination. Due to these limitations, there is a pressing need to explore new cathode architectures that support highly reversible redox reactions and maintain structural integrity over long-term use.
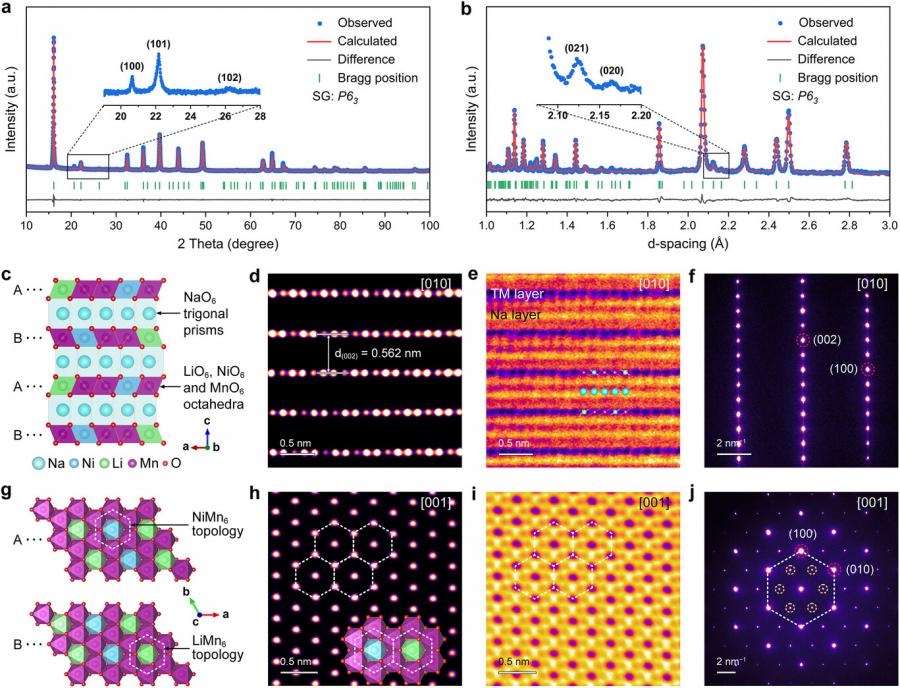
Published in Advanced Materials in April 2025, this collaborative work by researchers from Nankai University and Soochow University introduces a P2-type layered oxide cathode with a dual-topology NiMn₆/LiMn₆ superlattice. Through theoretical modeling, in situ X-ray techniques, and solid-state NMR, the team systematically investigated the structural and electrochemical behavior of the material. Their findings highlight how carefully tuned atomic configurations can suppress detrimental changes during cycling and enable balanced sodium storage mechanisms.
In the NNLMO cathode, Ni²⁺ acts as a redox buffer, while the NiMn₆ superlattice disperses the LiMn₆ domains, limiting manganese migration and oxygen release. This architecture significantly reduces voltage hysteresis—by up to 50% compared to conventional designs—and prevents O₂ gas evolution during operation. X-ray absorption spectroscopy and NMR confirmed the reversibility of both Mn/Ni and oxygen redox processes. DFT simulations revealed that Ni stabilizes the oxygen 2p orbitals and reduces Mn³⁺-induced distortions. The cathode maintains structural integrity across a wide voltage range (1.5–4.5 V) with only 1.5% volume change, far lower than in conventional materials. These features contribute to strong cycling performance, with over 92% capacity retention in half-cells and 84.7% in full-cell configurations.
“Our work illustrates the power of topological bi-superlattice engineering in resolving long-standing stability issues in oxygen-redox cathodes,” said Professor Fangyi Cheng, co-corresponding author of the study. “By integrating Ni-based redox buffering and a dual-honeycomb design, we achieved a level of redox symmetry and structural durability previously unattainable in sodium-ion systems. This strategy offers new design principles not just for SIBs, but also for broader multivalent and post-lithium battery chemistries.”
The introduction of a dual-topology superlattice represents an important step toward commercially viable sodium-ion batteries. By addressing critical challenges such as oxygen instability and structural degradation, the approach enhances energy density and cycle life, making SIBs more suitable for renewable energy storage and grid integration. Future research will focus on scaling the cathode design for large-format batteries and exploring its adaptability to other electrochemical systems.
References
DOI
10.1002/adma.202419137
Original Source URL
https://doi.org/10.1002/adma.202419137
Funding information
This work was supported by the China National Funds for Distinguished Young Scientists (21925503), the National Natural Science Foundation of China (21835004, 92372001, 92372203 and 52072186), the National Key Research and Development Program of China (2022YFB2402200), Open Foundation of Shanghai Jiao Tong University Shaoxing Research In stitute of Renewable Energy and Molecular Engineering (JDSX2023003), and the Fundamental Research Funds for the Central Universities of China (63241206 and 9242000710).
Lucy Wang
BioDesign Research
email us here
Distribution channels: Science, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release