Unlocking the role of Lcn2 in COVID-19 lung damage
GA, UNITED STATES, June 30, 2025 /EINPresswire.com/ -- A new study reveals that the protein Lcn2, secreted by lung macrophages, plays a central role in exacerbating severe pneumonia caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. Researchers developed a lethal pneumonia mouse model using an adapted viral strain and found that elevated levels of Lcn2 directly correlate with disease severity. Lcn2 not only enhanced inflammatory signaling and neutrophil adhesion but also disrupted endothelial barriers, leading to heightened lung damage. This research sheds light on a critical pathway—NLRP3-mediated Lcn2 secretion—that drives the escalation of inflammation in the lungs. The findings suggest Lcn2 as a potential diagnostic marker and therapeutic target for severe respiratory infections such as COVID-19.
Since the emergence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), understanding the mechanisms underlying severe pneumonia has remained a major research challenge. Although mouse models exist, most mimic only mild to moderate disease, limiting the ability to study life-threatening respiratory outcomes. Emerging data show that immune overactivation, especially through macrophage-driven inflammation, plays a decisive role in worsening COVID-19. Among many inflammatory mediators, lipocalin 2 (Lcn2) has been increasingly associated with respiratory disease severity, yet its regulatory mechanisms and pathological consequences are not well defined. Based on these challenges, there is a pressing need to investigate the upstream pathways and downstream effects of Lcn2 in virus-induced lung inflammation.
In a letter-style study published on August 24, 2024, in Protein & Cell, researchers from the Institute of Laboratory Animal Science, CAMS & PUMC, etc., reported that macrophage-secreted Lcn2 significantly worsens SARS-CoV-2-induced pneumonia in mice. By adapting the Beta variant to wild-type BALB/c mice, the team established a model of severe pneumonia, enabling detailed investigation into immune responses. The researchers identified Lcn2 as a key proinflammatory mediator activated through the NLRP3 signaling pathway, linking it directly to alveolar injury and systemic inflammation in viral lung infections.
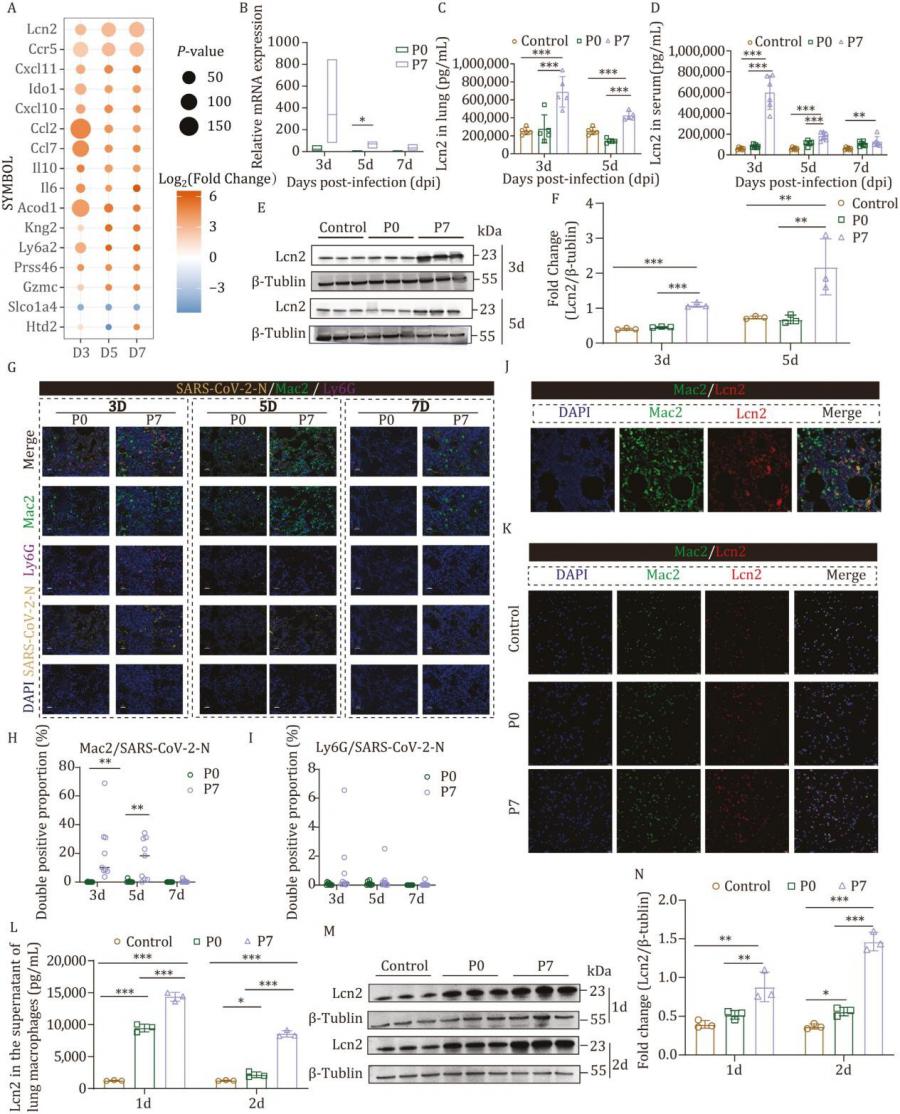
To create a more accurate model of severe COVID-19 pneumonia, the researchers developed a mouse-adapted SARS-CoV-2 strain (P7) that induced intense lung pathology and high mortality. P7-infected mice exhibited elevated levels of inflammatory cytokines and profound lung tissue damage. Transcriptomic and proteomic analyses revealed that Lcn2 expression was markedly upregulated following P7 infection. Further studies showed that macrophages were the main source of Lcn2, and that its production was driven by the NLRP3 signaling pathway. Inhibiting NLRP3 significantly reduced Lcn2 levels, confirming the pathway's central regulatory role. Functionally, Lcn2 was shown to amplify pulmonary inflammation by stimulating endothelial cells to express adhesion molecules (e.g., VCAM1), increasing neutrophil adhesion to endothelial cells, and weakening intercellular junctions. This resulted in compromised vascular integrity and greater immune cell infiltration. The team also discovered that a specific W682R mutation near the furin cleavage site in the viral spike protein may contribute to the enhanced infectivity and inflammation observed in the P7 strain. These findings provide crucial mechanistic insights into how viral evolution and host immune responses interact to produce severe lung pathology.
This study identifies Lcn2 as a key inflammatory mediator that drives severe lung damage during viral infection, said Dr. Linlin Bao, corresponding author of the study. By establishing a wild-type mouse model that closely mimics severe pneumonia, the researchers were able to uncover how the NLRP3-Lcn2 axis contributes to the pathogenesis. This opens new doors for understanding disease mechanisms and for targeting inflammation at its source, potentially leading to novel treatment strategies for severe COVID-19 and related respiratory diseases.
The discovery of Lcn2's central role in promoting severe pneumonia has broad implications. It positions Lcn2 not only as a biomarker for early detection of disease severity but also as a candidate for therapeutic intervention. Targeting the NLRP3-Lcn2 axis may offer a new strategy to mitigate lung injury in severe respiratory infections. Moreover, the established mouse model provides a valuable tool for testing antiviral and anti-inflammatory treatments. As new SARS-CoV-2 variants continue to emerge, understanding host-pathogen interactions like these will be critical for preparing for future public health threats.
References
DOI
10.1093/procel/pwae045
Original Source URL
https://doi.org/10.1093/procel/pwae045
Funding information
This work was supported by the National Research and Development Project of China (grant no. 2023YFF0724800), the CAMS Initiative for Innovative Medicine of China (grant no. 2021-I2M-1-035), the Sector Fund (2060302), and Young Elite Scientists Sponsorship Program by CAST (YESS) (grant no: 2020QNRC001).
Lucy Wang
BioDesign Research
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release